Write the Full Ground-state Electron Configuration for Each Element.
Write the full ground-state electron configuration for each element. Write the full ground-state electron configuration for each.
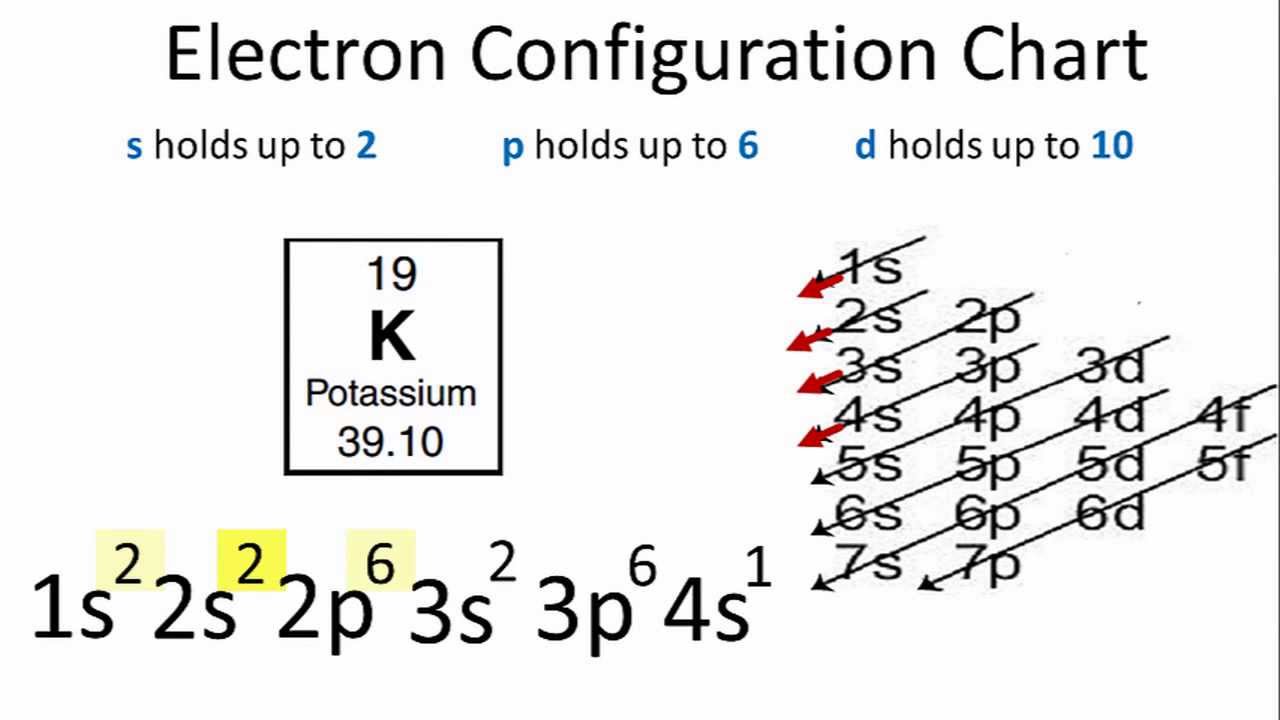
Electron Configuration For Potassium K
Rubidium has the atomic number 37.

. Electron Configuration of Oxygen. Um and today Im helping you with a problem. We review their content and use your feedback to keep the quality high.
Bromine with atomic number 35 belongs to group seven 7 period four 4 it ground state electron configuration is 1s²2s²2p63s²3p⁶3d¹⁰ 4s² 4p⁵. Write the full ground state electron configuration for each element. Up to 24 cash back Electron Configuration Practice Chemistry Name.
View the full answer. Which neutral atom is isoelectronic having same electronic structure of es with F. Sodium iron bromine barium neptunium I 2s22p6 3sz SZ2s2 F.
The F orbital has maximum of 14 electrons. 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p5 Mg. Cobalt silver tellurium radium Clzr Ltd q 10.
This problem has been solved. Keep filling the orbitals until we reach 37. Our videos prepare you to succeed in your college classes.
Write the long version for the ground state electron configuration for each of the following elements. - 14654741 emilyhoo9935 emilyhoo9935 02042020 Chemistry College answered expert verified Write the full ground-state electron configuration for each. Sc Ar 3d1 4s2 Sc Ar 3d1 4s1 Sc2 Ar 3d1 The 3d and 4s orbitals are close in energythe energy difference is small enough that the effect of e--e-repulsion.
Magnesium with atomic number 12 belongs to group one period two 2 it ground state electron configuration is 1s2 2s2 2p6 3s². Write the full electron configuration for tellerium Te list the number of valence electrons this atom has arrow_forward Use the concepts of effective nuclear charge shielding and n value of the valence orbital to explain the trend in atomic radius. See the answer See the answer done loading.
A Br b Mg c Se. Keep in mind electron configurations are most stable when they are filled or half. A Rb b Ge c Ar.
The concept of electronic configuration has replaced the older concept of valency and valence electrons. Write the full ground-state electron configuration for each. For example Rubidium will change from 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 5s1 to 1s2 2s2 2p6 3s2 3p6 4s2 3d10 4p6 because it loses the last electron.
Ground state configuration of. Long way We know that Krypton has 36 electrons. To write the electron configuration you start with the normal electron configuration of the element and add or subtract the number of electrons that the charge indicates.
Rn the space below write the Noble Gas abbreviated of the following elements. The electronic configuration of each element is decided by the Aufbau principle which states that the electrons fill orbitals in order of increasing energy levels. Write the full ground-state electron configuration for each.
Write the full ground state electron configuration of F. Write the full ground-state electron configuration for each element. Be sure to answer all parts.
We know that the spd orbital can hold a max of 2610 electrons respectively. Alternatively write the symbol for the noble gas before an element radon in this case and just add the extra information. Our videos will help you understand concepts.
1s 2 2s 2 2p 6 3s 2 3p 6 4s 2 3d 10 4p 6 5s 2 4d 10 5p 6 6s 2 4f 14 5d 10 6p 6 7s 2 5f 14 6d 10 7p 6. There is no one single or best structure for the periodic table but by whatever consensus there is the. Since the number of protons and electrons are equal we can identify the electron configuration by filling the orbitals with 37 electrons.
The ground state electronic configurations of the 0 1 and 2 oxidation states of the element scandium Sc provide a really good example for this argument. In the space below write the full unabbreviated electron configurations of the following elements. Seventh edition on and the problems Just asking us to write down electron configurations about several elements.
Previous question Next question. Using this as a. I will go through both methods.
My name is Margaret. Write the long version for the ground bartleby. 25 from chemistry the molecular nature of matter and change.
So if you want to know uh want to see a more in depth working out of how exactly I go through the process of doing this quickly. One way is to write out the entire electron configuration by going through each orbital or we can use a shorthand notation using the noble gases as a starting point. Rn 5f 14 6d 10 7s 2 7p 6.
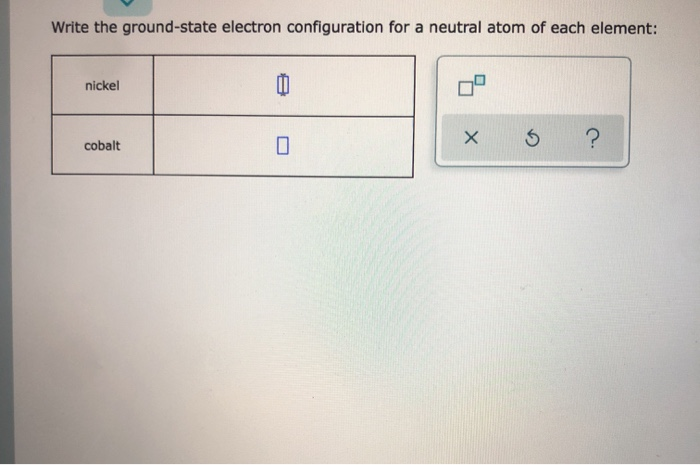
Let us help you simplify your studying. 100 6 ratings Transcribed image text. Write the full ground-state electron configuration for each element.
A Rb b Ge c Ar 1 See answer Advertisement. This is the best answer based on feedback and ratings. 119 rows Its electron configuration is.
If you are having trouble with Chemistry Organic Physics Calculus or Statistics we got your back. Find step-by-step Chemistry solutions and your answer to the following textbook question. Write the full ground-state electron configuration for each element.
Solved Write The Ground State Electron Configuration For A Chegg Com

Electron Configurations How To Write Out The S P D F Electronic Arrangements Of Atoms Ions Periodic Table Oxidation States Using Orbital Notation Gce A Level Revision Notes

6 4 Electronic Structure Of Atoms Electron Configurations Chemistry

Yttrium Y Electron Configuration And Orbital Diagram
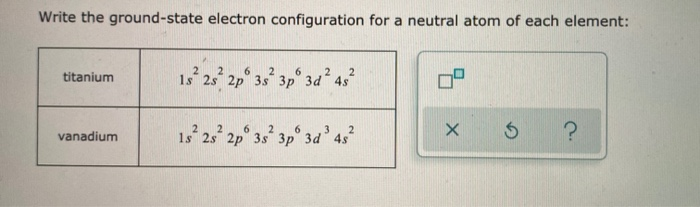
Solved Write The Ground State Electron Configuration For A Chegg Com
What Is The Electron Configuration For I Quora
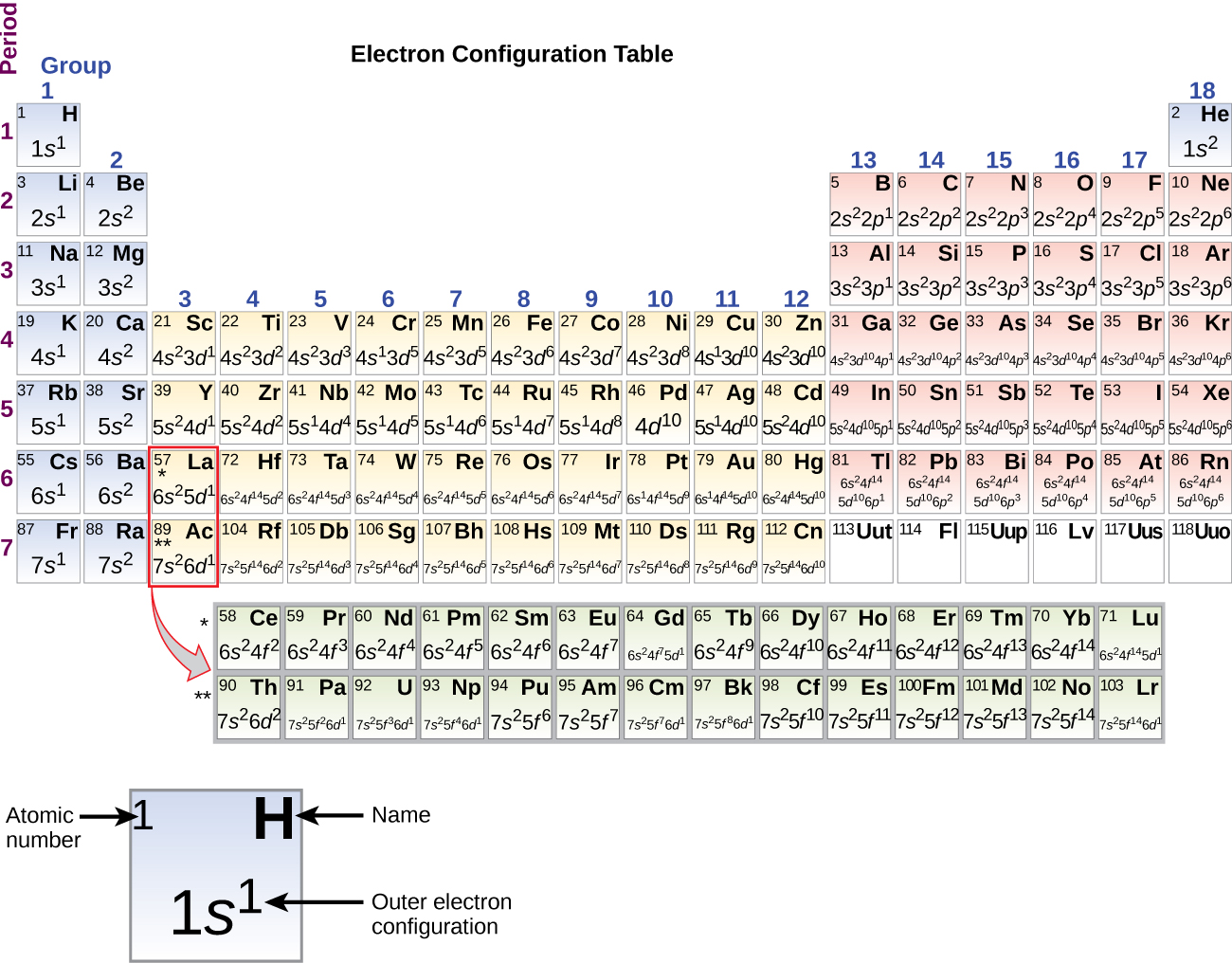
3 1 Electron Configurations Chemistry Libretexts
Solved Write The Ground State Electron Configuration For A Chegg Com
Free Solution Give The Ground State Electron Configuration For Each Of The Following Elements A Oxygen

Electron Configuration How To Identify The Element Youtube

Electron Configuration Of Ions Mg2 P3 Fe2 Fe3 Youtube
Electron Configuration Quick Review Youtube
S 2 Electron Configuration Sulfide Ion Youtube

Electron Configurations For The Second Period Video Khan Academy

Electron Configuration For Cu Cu And Cu2 Copper And Copper Ions Youtube


Comments
Post a Comment